SEATTLE — Teenagers and young adults should get new vaccines to prevent potentially deadly meningitis B infections, but only through individual decisions, not routine recommendations, a federal panel of experts decided Wednesday.
The 14-1 vote by Advisory Committee on Immunization Practices falls short of the broad recommendation urged by parents whose children have died from the disease and victims left disfigured or disabled. They said they feared the limited advisory will curtail wide access to the lifesaving shots.
“I field calls from parents all over this country who can’t get their hands on this vaccine,” Alicia Stillman, a Michigan mother whose 19-year-old daughter, Emily Stillman, died in 2013 from a fast-moving meningitis B infection, told the committee. “In 2015, we’ll become a community of haves and have-nots.”
But the ACIP panel, which advises the Centers for Disease Control and Prevention, agreed that historically low levels of the disease, limited data about the lasting effectiveness of the vaccines and potentially high costs didn’t warrant the wider recommendation.
ACIP members approved a category B, or “permissive,” recommendation that says teens, along with their doctors and families, can get meningitis B vaccines between ages 16 and 23. They declined to approve a category A recommendation for wide use, but group members said the vaccines could appear on a list of shots relied on by doctors and parents.
Carl Buher, a 26-year-old Seattle man who lost three fingers and both legs below the knee to a meningitis B infection as a teen, called the lesser decision “disappointing.”
But he and others affected by the disease will continue to advocate for wider use, he said.
“We’ll keep working until we get it to that higher level of recommendation,” he said.
There are about 50 to 60 cases of meningitis B in adolescents and young adults in the U.S. each year, including between five and 10 deaths, data show. Between 1 million and 3 million people would need to be vaccinated to prevent a single death, scientists said Wednesday.
Vaccines are already routinely recommended for college-age students to prevent four other strains of meningitis, but not strain B.
Bacterial meningitis is a rare infection that attacks the linings of the brain and spinal cord. It’s typically spread by close contact through respiratory secretions, and older teens and young adults are particularly at risk.
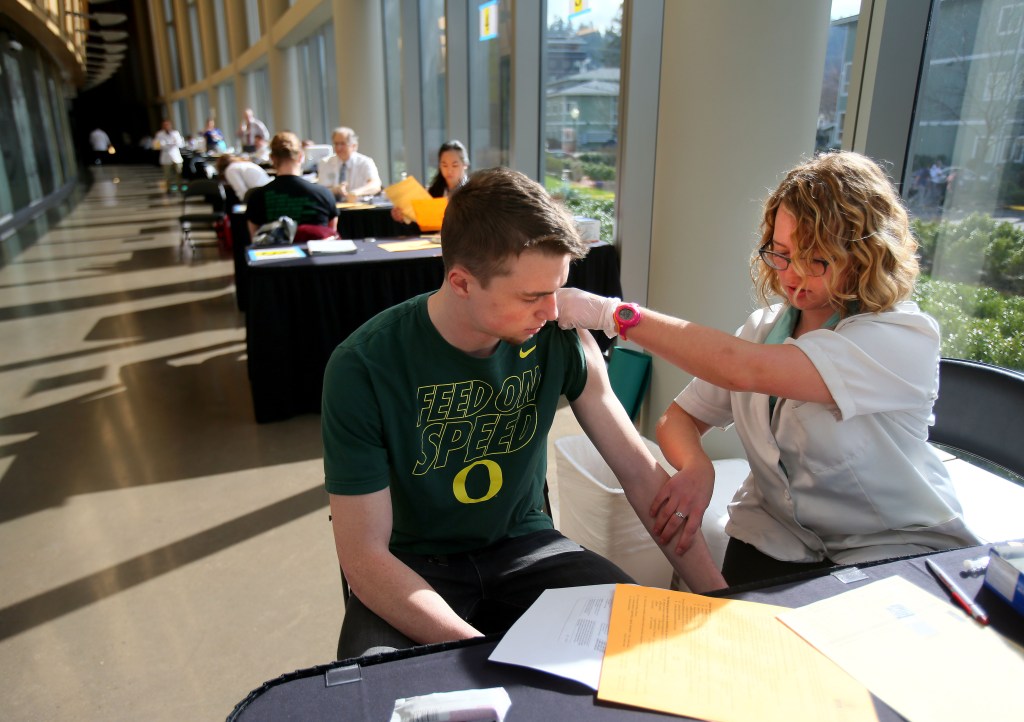
The decision follows recent outbreaks of meningitis B infections on college campuses, including Princeton University, the University of California, Santa Barbara and, this year, the University of Oregon, where seven people have been infected and a student died.
Under the new recommendation, two newly licensed meningitis vaccines — Trumenba from Pfizer and Bexsero, now offered by GlaxoSmithKline — will be offered under “permissive” guidelines. That means the shots can be covered by private insurance and the federal Vaccines for Children program.
Send questions/comments to the editors.


Success. Please wait for the page to reload. If the page does not reload within 5 seconds, please refresh the page.
Enter your email and password to access comments.
Hi, to comment on stories you must . This profile is in addition to your subscription and website login.
Already have a commenting profile? .
Invalid username/password.
Please check your email to confirm and complete your registration.
Only subscribers are eligible to post comments. Please subscribe or login first for digital access. Here’s why.
Use the form below to reset your password. When you've submitted your account email, we will send an email with a reset code.