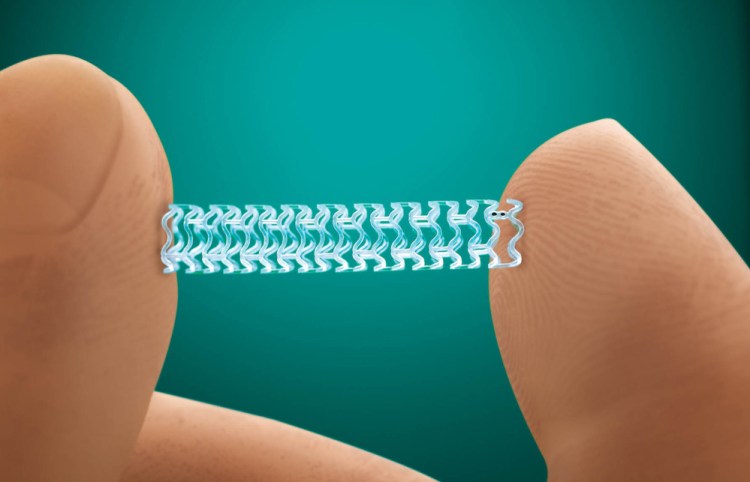
CHICAGO — Abbott Laboratories has a new trick up its sleeve: the disappearing heart stent.
The U.S. Food and Drug Administration has approved the company’s Absorb stent, which is designed to dissolve after treating a blocked blood vessel, Abbott said Tuesday. The technology is the first of its kind in the U.S.
“The Absorb bioresorbable stent treats coronary artery disease without committing people to a permanent metal implant – giving them peace of mind and helping them get back to their daily lives,” Deepak Nath, senior vice president in Abbott’s vascular division, said in a news release. “We’re very excited to bring the promise of Absorb to patients in the United States.”
The device, already sold in more than 100 countries, has the potential to improve the fight against heart disease by reducing the body’s exposure to foreign substances. Stents are tiny mesh tubes designed to prop open clogged arteries. Traditional metal stents restrict vessel movement and limit future treatment options. Absorb is made of a degradable material, similar to disappearing medical stitches, that slowly disappears in about three years, Abbott said.
Absorb performed as well as a drug-coated metallic stent, also made by Abbott called Xience, in a human clinical trial of about 2,000 patients. But the study also found a slightly higher incidence of blood clotting, or thrombosis, in patients who received Absorb, which makes some cardiology researchers wary.
An FDA advisory panel overwhelmingly endorsed the dissolving stent in March, leading to regulatory approval. Dr. John Somberg of Rush University Medical Center in Chicago, who was on the advisory panel and voted in favor of Absorb, said the benefits outweighed the risks.
“The panel felt this could be a game-changer in terms of technology, and it performed essentially the same as the best in the field,” Somberg said.
Patients who receive metallic stents have to take drugs to protect them from blood clotting. With Absorb, the hope is that patients will not have to take the anti-clotting drugs long term, Somberg said.
Coronary heart disease is responsible for about 370,000 deaths each year in the U.S., according to the National Heart, Lung, and Blood Institute. Heart stents are used on about 850,000 patients each year in the U.S. alone, according to The Associated Press.
The coronary stents market is largely dominated by a few key players, including Abbott, Boston Scientific and Medtronic. Abbott’s sales of coronary devices, which includes other products, fell 7 percent last year because of the negative effects of a strong U.S. dollar. Excluding foreign currency effects, sales rose 1 percent.
Abbott declined to disclose Absorb’s price. A company spokesman said Absorb will be modestly higher priced than the company’s conventional stents.
Send questions/comments to the editors.


Success. Please wait for the page to reload. If the page does not reload within 5 seconds, please refresh the page.
Enter your email and password to access comments.
Hi, to comment on stories you must . This profile is in addition to your subscription and website login.
Already have a commenting profile? .
Invalid username/password.
Please check your email to confirm and complete your registration.
Only subscribers are eligible to post comments. Please subscribe or login first for digital access. Here’s why.
Use the form below to reset your password. When you've submitted your account email, we will send an email with a reset code.