PORTLAND — Biotechnology firm ImmuCell makes two big batches of a cheddar-like cheese every week in a laboratory in Portland.
But you wouldn’t want to eat ImmuCell’s cheddar.
It’s made from cow colostrum, nutrient-rich milk that mammals produce when birthing.
Apparently it’s not very tasty.
But that’s OK with ImmuCell, because the cheese is a byproduct in the production of whey, an antibody-rich substance used by farmers to prevent disease in newborn calves.
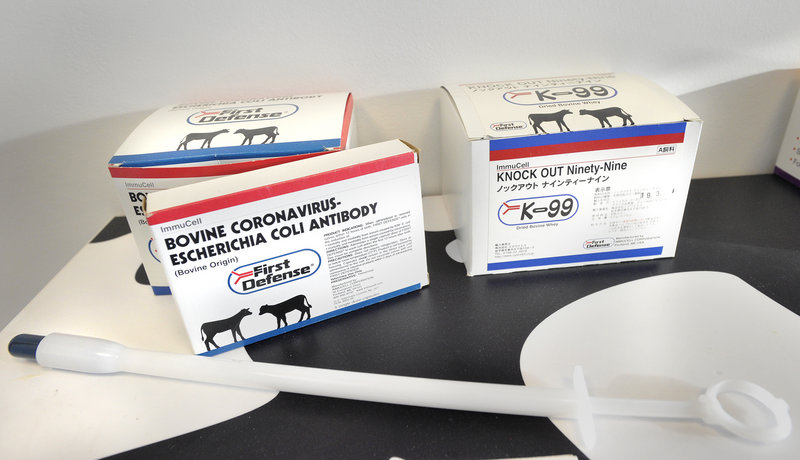
For 20 years, ImmuCell has been selling whey, in capsule form, to domestic and international dairy farmers. Demand for the product, called First Defense, has been strong, even during the recession. And the revenue from the product has allowed ImmuCell to invest millions of dollars – and with relatively low risk – in the development of a new product called Mast Out.
Mast Out still must be approved by the Food and Drug Administration, but insiders say its huge sales potential could give ImmuCell a notable competitive advantage and launch the relatively tiny Maine-based company into the top tier of biotech firms.
First Defense is given to newborn calves to prevent diseases like scours, which causes diarrhea and dehydration.
In a nondescript, second-floor boardroom at ImmuCell’s Evergreen Drive headquarters, President and CEO Michael Brigham proudly displays a foot-long plastic device resembling an oversized syringe that farmers use to administer the blue bullet-sized capsule in the throats of the newborn calves.
First Defense was the first major commercial success for ImmuCell, which was founded in 1982 by Tom Adelman and Frank Ruch, alumni of Ventrex, a biotech firm once based in Portland.
ImmuCell went public in 1987 and received U.S. Department of Agriculture approval to sell First Defense in 1991. The company is one of a handful of biotechnology companies, including Idexx Laboratories, that call the Portland area home.
Since then, 10 million First Defense doses have sold, most in the United States, but also in Canada, Japan, South Korea and Mexico. Each dose costs $6.
Candice Dotterer, dairy manager at Paul Dotterer & Sons Dairy Farm in Mill Hall, Pa., has been treating heifer calves with First Defense for four or five years. The product, she says, noticeably improved the health of her 800-cow herd.
“We had problems with respiratory issues and pneumonia, and once we started with First Defense, a lot of those problems decreased,” she said.
First Defense is ImmuCell’s best-selling product, but the company has always sought to diversify.
In the 1990s, ImmuCell researched human applications for its antibody products, a venture later discontinued.
Over the years, the company acquired products to prevent, test and treat the udder infection mastitis, including Wipe Out Dairy Wipes, which kill mastitis-causing bacteria when applied to the outside of the udder.
In 2000, ImmuCell, initially in partnership with Pfizer, began work on Mast Out, a product that treats mastitis inside the udder.
Other companies sell internal treatments for mastitis, including industry giants Pfizer Animal Health, Intervet / Schering-Plough Animal Health and Boeringher Ingelheim.
But Brigham said all those companies’ products have one major drawback – the FDA requires milk from cattle treated with them to be discarded.
That leaves farmers with two choices: spend money on treatment, or send the cows straight to the slaughterhouse, Brigham said.
ImmuCell is banking on the FDA approving Mast Out without the so-called “milk discard requirement.”
If that happens, Mast Out could be the only product of its kind and could save farmers billions in lost revenue, according to Brigham.
“There is no other product like that,” he said. “We would really compete with Pfizer, and have a competitive advantage.”
ImmuCell has been developing and testing Mast Out for 10 years, and the company has partnered with Swiss pharmaceutical company Lonza to produce an ultra-pure form of nisin, the key chemical in Mast Out.
Brigham, who expects an FDA ruling by next year, said approval is uncertain. Although ImmuCell has some patents, he said nisin has been around for years and other, larger companies have access to it.
ImmuCell has the advantage of being ahead, Brigham said, both in the tricky production of pharmaceutical-grade nisin and the lengthy FDA approval process, which is nearly complete.
If the FDA approves Mast Out, ImmuCell will have three years of market exclusivity.
One of ImmuCell’s largest investors, Sam Rebotsky of Forest Hills, N.Y., said he sees “the possibility of a home run with Mast Out.”
But Rebotsky, an investment adviser and accountant who owns 4 percent of ImmuCell stock, said development has taken longer than he anticipated.
“I thought this would move faster, so they wouldn’t need to spend as much money,” he said.
The company spent nearly $5 million on product development between 2007 and 2009, and another million in the first nine months of 2010, according to Securities and Exchange Commission filings.
Rebotsky also thinks ImmuCell has been a bit too quiet in communicating Mast Out’s potential.
“I had hopes they had been more prone to tell their story – to let people know what they are doing,” he said. “You need to be able to tell your story so you can attract additional funds and build a larger company.”
Brigham said hyping Mast Out might boost the stock temporarily, but will not affect the company’s long-term worth.
“The business we are building will ultimately determine the value of this company,” he said.
Brigham added that ImmuCell has limited resources. The company decided to invest in the business rather than investor relations.
“You have to make choices to where you allocate resources,” Brigham said. “We allocate them towards selling First Defense and developing Mast Out.”
ImmuCell is tiny compared to competitors.
The company, which trades on the Nasdaq market under ICCC, has 32 staffers and a market capitalization of just $9.47 million. comparison, Pfizer’s market capitalization is $148 billion.
ImmuCell reported annual profits every year for nine years starting in 1999. In 2007, the company made $662,000.
But starting in 2008, Mast Out development costs – and the recession – led the company to losses of $469,000. ImmuCell lost $216,000 in 2009 and $257,000 in the first three quarters of 2010.
But Brigham expects ImmuCell to be in the black by 2012, when Mast Out development ceases.
Even if it’s not approved, he said the company can stay profitable with First Defense sales.
Rebotsky, the investor, agrees.
“The FDA may not approve this product. It’s not a layup,” he said. “(But) if they stop spending on research and development, First Defense would be substantially profitable.”
Staff Writer Jonathan Hemmerdinger can be reached at 791-6316 or:
jhemmerdinger@mainetoday.com
Send questions/comments to the editors.



Success. Please wait for the page to reload. If the page does not reload within 5 seconds, please refresh the page.
Enter your email and password to access comments.
Hi, to comment on stories you must . This profile is in addition to your subscription and website login.
Already have a commenting profile? .
Invalid username/password.
Please check your email to confirm and complete your registration.
Only subscribers are eligible to post comments. Please subscribe or login first for digital access. Here’s why.
Use the form below to reset your password. When you've submitted your account email, we will send an email with a reset code.