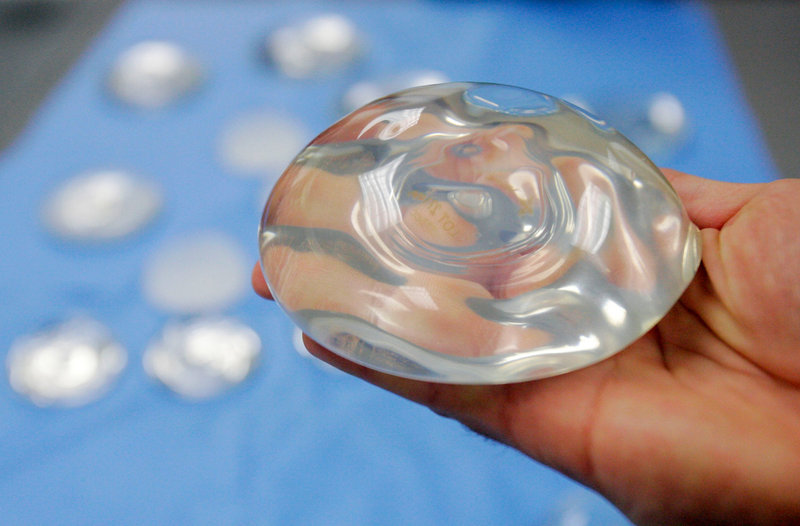
WASHINGTON – Don’t expect breast implants to last for life, the government warned Wednesday: About 1 in 5 women who receive them for cosmetic reasons will have them removed within 10 years, and those odds are even higher for cancer survivors.
It’s not the first time the Food and Drug Administration has issued such a warning. But the agency repeated it Wednesday after reviewing new data on silicone-gel breast implants five years after they returned to the market following a health scare. The agency concluded the implants are basically safe as long as women understand they come with complications. Those include painful scar tissue and ruptured implants.
“The longer you have the implant, the more likely you are to have complications,” said FDA medical device chief Jeff Shuren. He said women should get regular checkups including scans to make sure the implants haven’t ruptured.
While FDA’s safety review concentrated on silicone-gel implants, the agency’s updated advice booklet for women makes clear that saline-filled versions come with the same complications.
Plastic surgeons say they’ve long told women about those risks.
“It doesn’t discourage a single one of them, which is pretty amazing,” said Dr. Michael Zenn, vice chief of plastic surgery at Duke University Medical Center. “This requires almost lifetime maintenance when you have a breast implant in. If you’re not telling patients that, you do them a disservice.”
Wednesday’s update is the latest in a 20-year saga over the safety of breast implants. The FDA banned the silicone-gel type in 1992 amid fears they might cause cancer, lupus and other diseases. But when research ruled out most of the disease concern, regulators returned the implants to the market in 2006 — with the requirement that manufacturers continue studying recipients to see how they fare long-term.
Breast augmentation remains the most popular cosmetic surgery in the U.S., with nearly 300,000 women undergoing it last year. According to the American Society of Plastic Surgeons, more than 70,000 others received implants for breast reconstruction. Silicone-gel implants are the most common kind.
Based on that data, FDA said Wednesday that 20 percent to 40 percent of patients who have implants for cosmetic reasons will need another operation to modify or remove them within eight to 10 years.
For reconstruction patients, the number is even higher at 40 to 70 percent, FDA said.
The most common complication remains scar tissue that hardens around the implant, and that can become severe enough to warp the shape of the breast or cause pain. Other problems include implant rupture, wrinkling and a lopsided appearance, according to the report.
Copy the Story Link
Send questions/comments to the editors.


Success. Please wait for the page to reload. If the page does not reload within 5 seconds, please refresh the page.
Enter your email and password to access comments.
Hi, to comment on stories you must . This profile is in addition to your subscription and website login.
Already have a commenting profile? .
Invalid username/password.
Please check your email to confirm and complete your registration.
Only subscribers are eligible to post comments. Please subscribe or login first for digital access. Here’s why.
Use the form below to reset your password. When you've submitted your account email, we will send an email with a reset code.