WASHINGTON — The explosion of new food additives coupled with an easing of oversight requirements is allowing manufacturers to avoid the scrutiny of the Food and Drug Administration, which is responsible for ensuring the safety of chemicals streaming into the food supply.
And in hundreds of cases, the FDA doesn’t even know of the existence of new additives, which can include chemical preservatives, flavorings and thickening agents, records and interviews show.
“We simply do not have the information to vouch for the safety of many of these chemicals,” said Michael Taylor, the FDA’s deputy commissioner for food.
The FDA has received thousands of consumer complaints about additives in recent years, saying certain substances seem to trigger asthmatic attacks, serious bouts of vomiting, intestinal-tract disorders and other health problems.
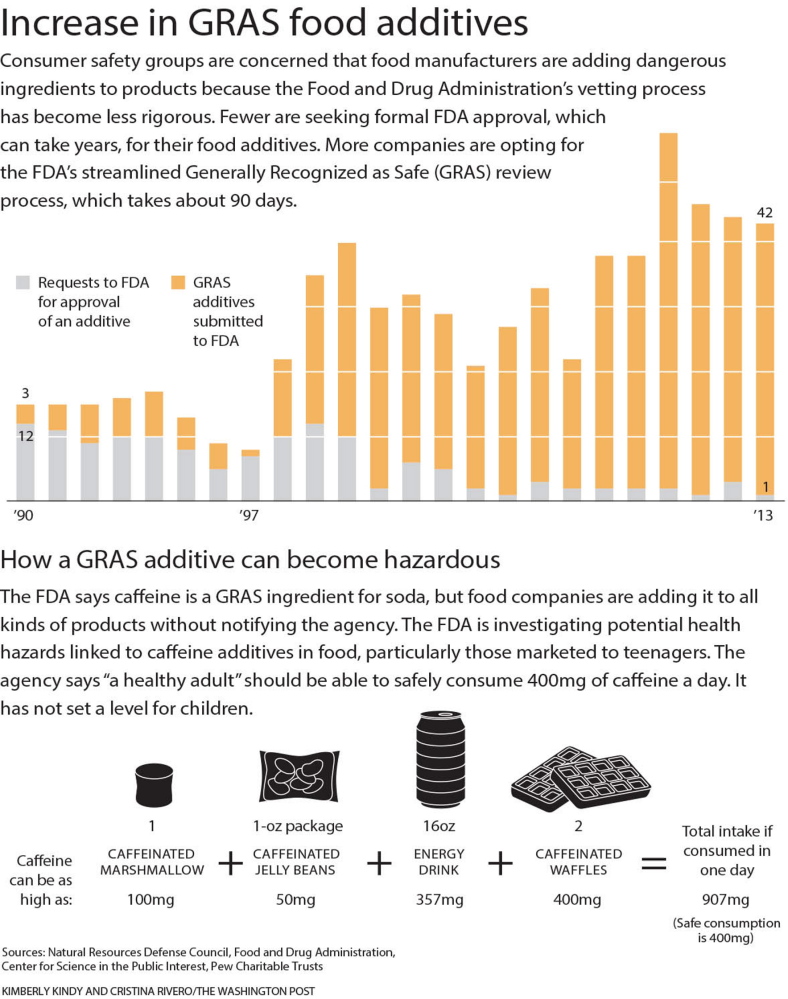
At a pace far faster than in previous years, companies are adding secret ingredients to everything from energy drinks to granola bars. But the more widespread concern among food-safety advocates and some federal regulators is the quickening trend of companies opting for an expedited certification process to a degree never intended when it was established 17 years ago to, in part, help businesses.
A voluntary certification system has nearly replaced one that relied on a more formal, time-consuming review – where the FDA, rather than companies, made the final determination on what is safe. The result is that consumers have little way of being certain that the food products they buy won’t harm them.
“We aren’t saying we have a public health crisis,” Taylor said. “But we do have questions about whether we can do what people expect of us.”
THE NUMBER OF ADDITIVES EXPLODES
In the five decades since Congress gave the FDA responsibility for ensuring the safety of additives in the food supply, the number has spiked from 800 to more than 9,000, ranging from common substances such as salt to new green-tea extracts.
This increase has been driven largely by demand from busy Americans, who get more than half their daily meals from processed foods, according to government and industry records.
Within the past six months, top officials at the FDA and in the food industry have acknowledged that new steps must be taken to better account for the additives proliferating in the food supply.
The Center for Food Safety, an advocacy group, has responded more aggressively; it sued the FDA this year, saying the agency has abdicated its oversight of the additives approval process.
The Grocery Manufacturers Association also provided seed money this spring to create a research center at Michigan State University to deal with the rising concerns over additives.
For new, novel ingredients – or when approved additives are used in new ways – the law says companies should seek formal FDA approval, which must be based on rigorous research proving the additive is safe. The agency uses the phrase “food additive,” in a narrow legal sense, to apply to substances that get this approval.
VINEGAR AS AN EXAMPLE
But many other additives are common food ingredients – vinegar is considered a classic example. The law allows manufacturers to certify, based on research, that such ingredients are already Generally Recognized as Safe, or GRAS.
For both types of additives, FDA scientists initially conducted detailed reviews of the company’s research. The agency also published its own evaluation of that research in the Federal Register.
This oversight system shifted dramatically in 1997. In response to a shortage of staff members and complaints from industry that the process was too cumbersome and did not improve food safety, the FDA proposed new rules. The agency told companies that were going the GRAS route – which turned a years-long process into one of months – that they no longer would have to submit their research and raw data. The companies can share just a summary of their findings with the agency.
In part, FDA officials hoped that by streamlining the GRAS notification process, companies that previously avoided informing the agency of new additives would be encouraged to keep the government in the loop, current and former agency officials said.
The changes didn’t work out as planned.
For starters, most additives continued to debut without the FDA being notified.
Moreover, companies that did choose to go through the FDA oversight process largely abandoned the formal approval route, opting instead for the new, cursory GRAS process, even for additives that could be considered new and novel, according to agency documents and an analysis of those records by the Natural Resources Defense Council.
“They created a side door,” said Tom Neltner, a chemical engineer with the NRDC who has co-authored six academic articles about the FDA additives process over the past four years.
FEW UP FOR FORMAL APPROVAL
An average of only two additive petitions seeking formal approval are filed annually by food and chemical companies, while the agency receives dozens of GRAS notifications, according to an NRDC analysis of FDA data.
Hundreds of other food chemicals and ingredients have been introduced without notifying the FDA at all, according to agency officials, trade journals and food safety groups.
FDA officials, food safety advocates and the food industry all agree there are problems. There are too many cases in which the agency is not notified of new additives or the science remains secret. But there’s no consensus about how to fix the system.
Industry trade groups say that the additives in today’s foods do not pose a public safety risk, but most agree improvements for better tracking and oversight are needed.
“It’s the right time to take a step back,” said Leon Bruner, the Grocery Manufacturers Association’s chief science officer. “There are problems with transparency. How can we be sure that the FDA is aware of ingredients?”
When the manufacturer of a vegetarian line of foods called Quorn first approached the FDA in 1986, the company asked that the agency give formal additive approval for a protein-rich fungus the company makes in large fermentation vats.
Marlow Foods dubbed its new fungal ingredient “mycoprotein.”
Fifteen years passed without the FDA moving on the petition. Internal FDA documents, obtained through a Freedom of Information Act request, show no sign of the agency evaluating the safety claims made in the petition. Because the FDA declined interview requests about Quorn products, it is unclear why the petition sat for so long.
After the long delay and without withdrawing the original petition, Marlow filed a GRAS notification with the FDA in 2001, saying the firm had hired a group of experts who had determined that mycoprotein was safe. Under the rules, the notification did not – nor did it have to – cite every study the company had conducted.
ONE STUDY NOT MENTIONED
A study that was not mentioned was an unpublished “large scale volunteer trial” from the late 1970s. During the trial, nearly 5 percent of the 200 participants reported feeling ill after eating several “test meals,” according to the study, which was obtained by The Washington Post. Four had “more severe reactions,” including two who started to “vomit violently,” another who became “violently sick” and another who “experienced nausea and vomiting” after eating Quorn products that contain mycoprotein. The study says that the “reactions” could have been caused by a response to mycoprotein.
In a prepared statement, Quorn said the study had been shared with the FDA but was not cited in the GRAS submission because “it was a small study and at that time nearly 25 years old and more recent data was relevant and important to the submission.” The statement also said “many research studies” had been undertaken, and all were shared with the FDA and other regulators.
Once Marlow filed its GRAS notification, the FDA acted quickly. Within six weeks, the FDA signed off on it, using its standard language, noting it was not vouching for the safety of mycoprotein and that it was the “continuing responsibility of Marlow to ensure that food ingredients that the firm markets are safe.”
Within months, the Center for Science in the Public Interest began alerting the FDA to complaints from consumers in the United States and Britain, where the products have been sold since 1985.
“They said they broke out in hives and had breathing difficulties – anaphylactic reactions,” said Michael Jacobson, the group’s executive director. “The GI episodes they described were violent. They would vomit so hard it could break the blood vessels in their eyes.”
Independent researchers also published three papers in academic journals, between 2003 and 2009, describing severe and even life-threatening allergic reactions to mycoprotein.
The FDA ultimately contacted hundreds of people that the advocacy center sent their way. In 2012, the agency wrote to the group agreeing that “individuals may experience adverse reactions” to Quorn products and that a “food allergy cannot be ruled out.” However, FDA officials said they believed “most of these reactions are due to non-life threatening food intolerance.”
The FDA said it would not challenge Marlow’s GRAS findings.
AGENCY CRITICIZED
The CSPI said the FDA treated the concerns as an inconvenience more than a safety concern. It said the agency, at a minimum, should have issued a public warning to people who could be allergic or intolerant, or mandate warning labels. FDA officials strongly disagreed with this characterization, documents show.
In a statement to The Post, Marlow Foods said the fungal-based ingredient in its products is a “natural protein” that is “harvested and fermented in a similar way to beer, yeast or yogurt,” adding that claims that Quorn is unsafe “have been proven inaccurate and lack scientific credibility.” However, the company acknowledges on its website that some consumers may have a “true allergic reaction” to its products.
Companies often bypass the FDA altogether. Under the rules, companies may make their own GRAS determination. Sharing it with the agency and getting it to sign off is voluntary.
In 2007, Japan-based Kao Corp. tried to get the FDA to sign off on its GRAS determination for its extract containing the chemical Epigallocatechin-3-gallate, or EGCG. The company planned to promote its qualities as an antioxidant and possible fat-burner in a line of diet and sports drinks, according to FDA emails obtained by the Natural Resources Defense Council through a Freedom of Information Act request.
But the FDA toxicologists first asked Kao to address the findings of more than a dozen scientific studies, including one that showed it could induce “toxicity in the liver, kidneys and intestine.” Another showed it could produce “defects in the brain and heart.” And still another said it may “contribute to infant leukemia,” the FDA emails show.
FDA told Kao Corp. to go back to the drawing board. Instead, in 2008, it withdrew its notification. Kao spokeswoman Billie Cole said the company believes its products, which are marketed in Japan, are healthy and safe.
MARKETING GREEN-TEA POWDER
Three years later, another company, Iowa-based Kemin, took out advertisements in trade publications, announcing its green-tea powder containing EGCG was Generally Recognized as Safe. Kemin did not share its GRAS findings with the agency.
The company began marketing the substance to the food and beverage industry for use in “cereal, nutrition and energy bars, soft drinks, sports and isotonic drinks, energy beverages, fruit and vegetable juices, meal replacement and soft candies.”
In a prepared statement, Anita Norian, president of Kemin’s human nutrition and health division, said the product is safe and that the company chose “not to participate in the FDA’s voluntary GRAS determination notification process.” Norian said to do so would potentially undermine the company’s work, allowing other firms to discover “proprietary” information about its product.
This is the opposite of what the overight law intended, the FDA’s Taylor said. Although informing the FDA is voluntary, he said, the law was meant to increase public scrutiny of additive safety by encouraging companies to publish their science in academic journals.
“The assessments need to be based on publicly available information where there is agreement among scientists,” he said. “It has got to be more than three employees in a room looking at information that is only available to them.”
Even when the FDA approves a new additive or signs off on a company’s GRAS determination, a safe ingredient can turn dangerous if its use becomes more widespread than the agency envisioned. And under the rules, the agency has little way of monitoring this threat after the initial introduction of the additive, called “post-market.”
“We do not know the volume of particular chemicals that are going into the food supply so we can diagnose trends,” Taylor said. “We do not know what is going on post-market.”
During the initial review, the FDA sets limits for how much of a chemical or ingredient can be used in a particular product. But the cumulative consumption can soar as the additive is used in more and more types of food and beverages.
This is what happened with caffeine. In 1959, the FDA approved it as GRAS, allowing soft drink manufacturers to add it to their products. But now food manufacturers are loading caffeine into energy drinks, maple syrup, jelly beans and marshmallows.
And what happened with caffeine is now taking place with lesser-known additives.
CARRAGEENAN USE INCREASES
Carrageenan, extracted from red seaweed, was one of the first substances that the agency recognized as GRAS. The additive is a stabilizer that can help preserve the structure of foods and beverages and is used in products such as evaporated milk.
But as processed foods began to take off, and a demand for low-fat and tasty vegan processed foods became more popular, so did the use of carrageenan. A drop of it in a package of reduced-fat deli meat, a bundle of vegan cheese, a bottle of kefir or a box of almond milk can keep ingredients blended together and give products a pleasant texture.
Companies sought and secured the FDA’s approval for its use as a food additive for a wider variety of functions – including as a thickener and an emulsifier, which keeps ingredients bound together. That approval further restricted the type of red seaweed that could be used as the source.
Scientists working for the carrageenan industry say the additive is safe.
“When it’s bound to food protein, it cannot interact with the cells; it can’t interact with the intestinal wall,” said James McKim, president of the toxicological research firm CeeTox, who was recently hired as a consultant to the carrageenan trade group, Marinalg. “It runs right through the animals.”
But doctors say the proliferation of carrageenan in the food supply is taking a mounting toll on health. As its uses have multiplied, so have gastro-intestinal disorders such as diverticulitis, irritable bowel syndrome and Crohn’s disease, according to some doctors who specialize in treating patients with gastrointestinal tract problems.
Joanne Tobacman, a University of Chicago physician and professor, asked the FDA in 2008 to examine the additive’s safety, submitting five of her own studies concluding that carrageenan can cause inflammatory bowel disease and diabetes. The studies were financed with grants from the federal government.
The FDA denied her petition, citing several other industry-funded studies that contradicted her findings.
Melvyn Grovit, a clinical nutritionist who treats children with Crohn’s disease, said he started advising his clients to remove carregeenan from their diets with great success after reading Tobacman’s research.
“I’ve given up on the FDA protecting the public; they are protecting businesses,” said Grovit, who also suffers from Crohn’s disease. “The only reason for its presence in so many food products today is that it’s cheap and it makes food processing easy.”
Send questions/comments to the editors.



Comments are no longer available on this story